Q-SUPPORT® EXOSOMES
Extracellular vesicles purified from perinatal MSC lines, engineered as biological adjuvants in repair processes and inflammatory control. Its clinical application favors a more stable, functional and predictable tissue environment.
Presentation and concentration
Mechanism and evidence
They act as natural mediators of cellular communication, transferring proteins, miRNAs and bioactive factors that modulate the inflammatory response and stimulate endogenous regeneration mechanisms.
Promote the controlled angiogenesisthe reorganization of the extracellular matrix and the functional recovery of tissuereducing variability in clinical outcomes and return-to-work times.
Developed for scenarios where predictable recovery, pain reduction y sustained tissue stability during follow-up.
Origin and method
Source: perinatal MSC lines (placenta and umbilical cord) from donors selected under ethical and biosafety criteria.
Method of production: ultrafiltration + size exclusion chromatography (SEC) on a validated platform.
Cellular impurities: not detectable according to certificate of analysis (COA).
Characterization and quality
Id: expression of tetraspanins CD63, CD81 and CD9 (ELISA / Western Blot).
Physical-chemical analysis: count and size distribution by NTA; characteristic protein profile.
Microbiology: sterility (USP), mycoplasma (qPCR) and endotoxins (LAL).
Acceptance criteria: endotoxins < 0.5 EU/mL; all parameters within specification range per batch.
Use and preparation
Frequent clinical applications: orthopedics (intra-articular), dermatology and esthetics (intradermal), scar and lesion management (intralesional).
Routes of administration: intradermal, intra-articular or intralesional; intravenous use only under approved protocols.
Conservation and logistics
Storage: keep in cold net between 2 °C and 8 °C to its application.
Cold chain: do not administer if the product was out of the cold chain.
Preliminary inspection: shake gently the vial before use. Do not use if observed dark particles or if the solution is not homogeneous.
What are extracellular vesicles (exosomes)?
Subtype of vesicles of 30-150 nmdelimited by lipid bilayer and released by mesenchymal stem cells (MSC) characterized.
Transport proteins, lipids and nucleic acids (mRNA/miRNA) involved in intercellular signaling and regulation of the tissue microenvironment.
Recent literature supports its use as a cell-free strategywith low immunogenicity and potential in repair and inflammatory control protocols.
Cellular communication: modulate inflammatory and angiogenesis pathways.
Tissue repair: promote healing and structural remodeling processes.
Current evidence: preclinical studies and early clinical series in progress.
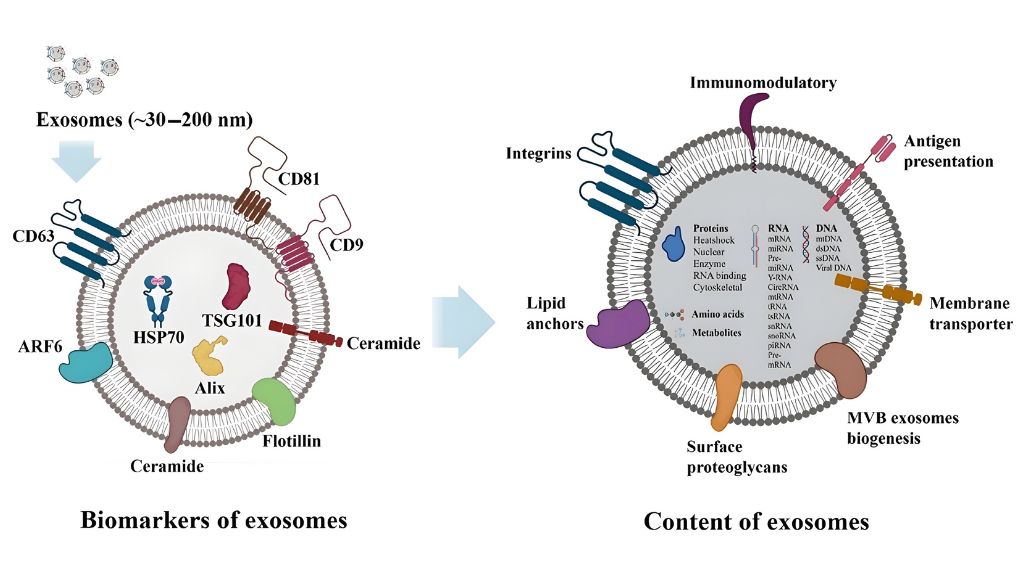
Hade MD, Suire CN, Suo Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells. 2021;10(8):1959. doi:10.3390/cells10081959. Image reproduced (via Macário-Soares A. et al., VIEW 2024; Fig. 2; doi:10.1002/VIW.20240043) under CC BY 4.0 license. The image was rescaled.
Actual useful dose (particles)
Actual useful dose (particles)
Particle count per presentation defines the biologically active dose. Reported by NTA (Nanoparticle Tracking Analysis) in each batch.
Documentation per lot
COA with NTA/BCA results, microbiology, endotoxins, traceability and identity markers (e.g., tetraspanins).
Declared content
Vial 5 mL - ≈ 7.5×10⁸ vesicles - 1,550 µg total protein (see COA).
Inter-lot reproducibility
Control of count and protein variability to ensure consistent dosing.
Our biotechnology products

Stromal Precursors (MSC)
10 Million
25 Million
50 Million
Mechanism and evidence
Paracrine and immunomodulation: factors and EV that adjust inflammation and promote repair.
Odontogenic/bony potential: differentiation odontoblast-like and bone; better performance with scaffolding adequate.
Regenerative endodontics: evolving protocols; results heterogeneous between studies.
Periodontium/implants: support in intrabony defects and ridge preservation (early evidence).
Natural Killer (NK) Cells
25 Million
50 Million
Mechanism and evidence
Direct cytotoxicity: cell recognition with MHC-I low/absent and lysis by perforin/granzymes.
ADCC (CD16): synergy with monoclonal antibodiesdestroys cells opsonized with IgG.
Immunoregulation: secretion of IFN-γ/TNF-α y crosstalk with dendritic and Tadjusting the tumor microenvironment.
Evidence framework: mechanisms valid in basic immunology; ongoing clinical translationwith response influenced by patient status, KIR/HLA profile and degree of activation ex vivo.
Exosomes
1,550 µg per vial of 5 mL
Mechanism and evidence
They act as natural mediators of cellular communication, transferring proteins, RNA and bioactive factors that modulate the inflammatory response and stimulate endogenous regeneration mechanisms.
Promote the controlled angiogenesisthe reorganization of the extracellular matrix and the functional recovery of tissuereducing variability in clinical outcomes and return-to-work times.
Developed for scenarios where predictable recovery, pain reduction y sustained tissue stability during follow-up.
